Abstract
Background Cancer therapy induced thrombopenia (CTIT) refer to thrombopenia caused by various anti-cancer therapy which include chemotherapy, radiotherapy, targeted therapy and immunotherapy. Chemotherapy-induced thrombopenia (CIT) could increase the risk of bleeding, modification of treatment, cost of treatment and even influence overall survival. Recombinant human thrombopoietin (rhTPO) was approved for CIT by national medical products administration (NMPA). However, certain patients still could not benefit from these agents. Hetrombopag, a new orally active small molecule thrombopoietin receptor agonist (TPO-RA), acts through binding to thrombopoietin receptor to stimulate multiple intracellular signaling pathways, including JAK/STAT, PI3K/AKT and ERK1/2. This will stimulate the proliferation and differentiation of megakaryocytes and promote platelet production. Combination of TPO-RA and rhTPO is anticipated to improve the outcome compared with rhTPO alone for treating CTIT in cancer patients.
Methods Between January 2021 and June 2022, we conducted a retrospective analysis of patients (18 - 80 years) with cancer therapy induced thrombopenia (platelet count < 50 x 109/L) treated with rhTPO (subcutaneous injection, 300 U/kg/d) alone or combination of rhTPO and hetrombopag (oral, 5 mg/d) at Beijing Luhe Hospital, Capital Medical University. Treatment will discontinue when platelet count increased more than 50 x 109/L or platelet count doubled than baseline. The maximum treatment was 14 days.
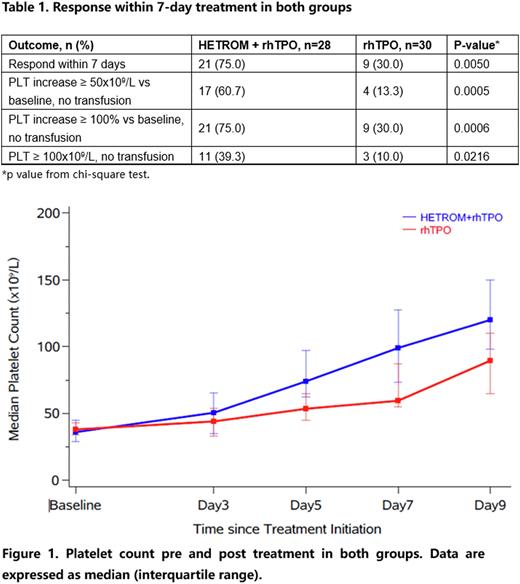
Results In total, 58 patients were included for analysis (28 patients received rhTPO plus hetrombopag and 30 patients received rhTPO alone). The baseline characteristics were generally similar between two groups. Patients with baseline platelet count less than 30 x109/L were 28.6% vs 10% in rhTPO plus hetrombopag group and rhTPO alone group, respectively. There were 21 patients (75.0%) vs 9 patients (30.0%) achieved response (platelet count increased more than 50 x 109/L or doubled than baseline or reached more than 100 x 109/L without platelet transfusion) within 7-day treatment in rhTPO plus hetrombopag group and rhTPO alone group, respectively. Furthermore, 11 patients (39.3%) and 3 patients (10.0%) reached platelet count ≥100 x 109/L in rhTPO plus hetrombopag group and rhTPO alone group (Table 1). The median platelet count at baseline in two groups were similar. The platelet count of post treatment in two groups were both increased. While the platelet count increase in rhTPO plus hetrombopag group were higher than rhTPO group in day 3, day 5, day 7 and day 9 after treatment (Figure 1). Median time of treatment was 6.5 days (range 4-13) for rhTPO plus hetromnopag group and 9.5 days (range 5-14) for rhTPO group (P < 0.0001). One patient (3.3%) in rhTPO group reported bleeding (WHO bleeding grade 2) while no bleeding events were reported in rhTPO plus hetrombopag group. Five patients (16.7%) and three patients (10.7%) in rhTPO group and rhTPO plus hetrombopag group received platelet transfusion.
Cancer patients treated with rhTPO or rhTPO plus hetrombopag were well tolerated, the treatment related adverse events were mainly alanine aminotransferase/aspartate aminotransferase increase or total bilirubin increased (all grade 1/2). The incidence of such adverse event was not significantly different between two groups.
Conclusion Compared with rhTPO, combined rhTPO with hetrombopag for treating cancer therapy induced thrombopenia showed faster and deeper response without raise the safety concern. Further study needed to consolidate this result.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal